The downside for growers is trying to keep these crops clear of pests and diseases. The diseases that cause problems for brassica growers in South Africa are many and varied in their causes, and in the past six years, another newcomer has been added to the list, in the form of Brassica Stunting Disease (BSD).
The Department of Agriculture, Forestry and Fisheries says diseases problematic to brassica growers include black rot (Xanthomonas campestris pv. campestris), Alternaria leaf spot (caused by a complex of three Alternaria species), white rust (whiteblister) (Albugo candida), downy mildew (Peronospora parasitica), black leg (Phoma lingam), club-root (Plasmodiophora brassicae), bacterial leaf spot (Pseudomonas syringae pv. maculicola) and Fusarium wilt (Fusarium oxysporum). The newest addition to this list is Brassica Stunting Disease (BSD), which first appeared in Brits in North West Province in 2012.
Since then the disease has been reported in all provinces except the Western Cape. Disease pressure for the above-mentioned list of brassica pathogens varies across the provinces, but industry representatives agree that BSD, along with whiteblister, clubroot and black rot, is one of the four most important diseases affecting brassica production in this country.
BSD affects various species, but cabbages (Brassica oleracea var. capitata) appear to be the most affected. The disease is characterised by stunted plants, flattening and sometimes purpling of the leaves, side shoot development, vascular discolouration in the stem and/or midrib of leaves and poor root development (fig. 1). Over the past six years, researchers at the University of Johannesburg have studied BSD and discovered that the disease is not seed, soil or water borne, but transmitted by various aphid species, including Myzus persicae (peach-potato or green-peach aphid).
Because there are no crop cultivars resistant to BSD yet, the study has turned its focus towards disease management strategies, and investigated the use of chemical control of the aphid vector. Findings of this research project were recently shared with growers during four BSD information days in July.
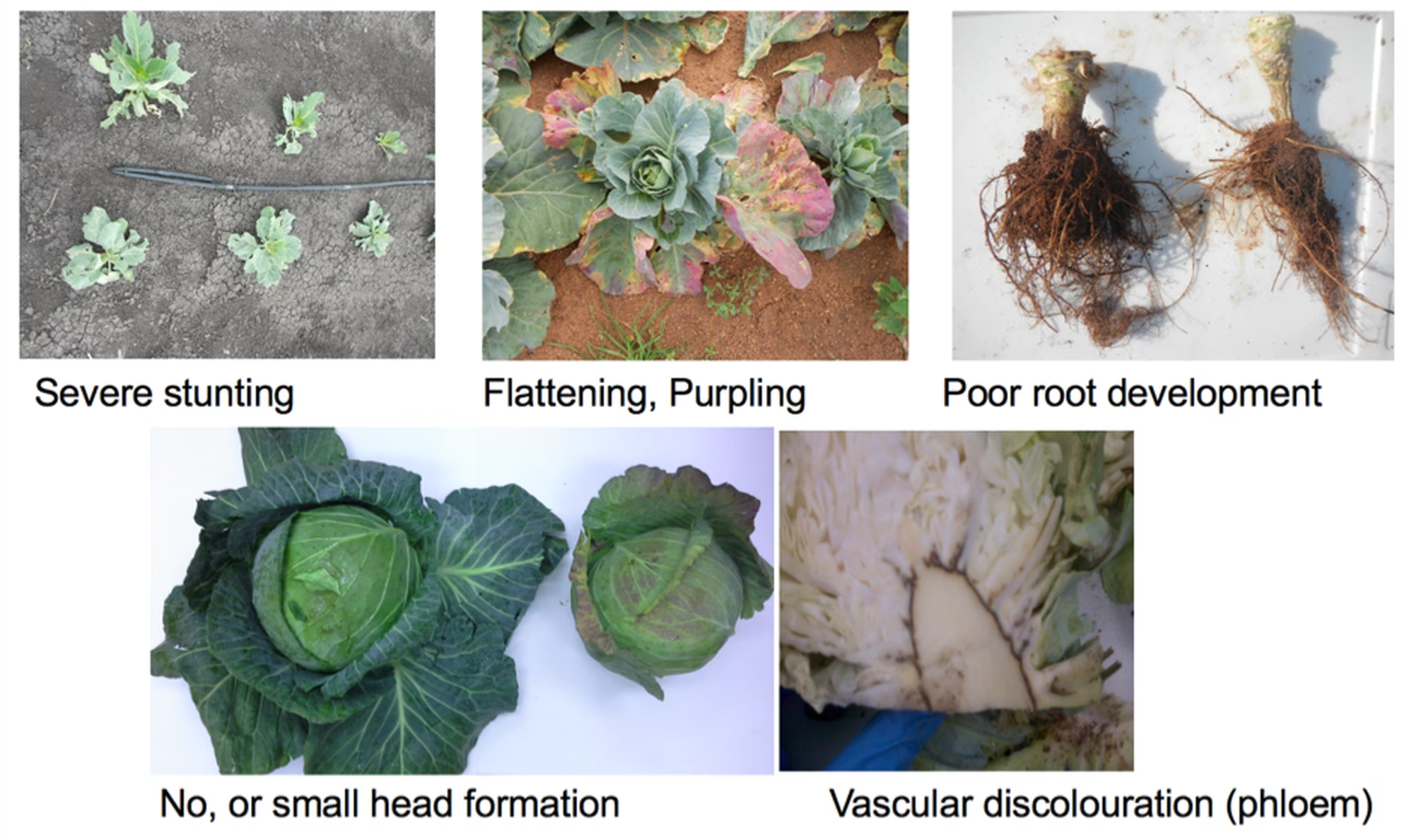
Figure 1. Disease symptoms of cabbage plants affected by BSD.
The BSD information days were held in different brassica production regions: Lydenburg (Mpumalanga), Bloemfontein (Free State), Hilton (KwaZulu-Natal) and Marble Hall (Limpopo). The events were organised and funded by Sakata, Starke Ayres, Syngenta, Bayer, the Seedling Growers Association of South Africa (SGASA) and McCain Foods. They were attended by both growers and roleplayers from the industry, including representatives from nurseries, agricultural chemical companies and seed companies, and well supported, with between 34 and 43 people attending the first three days.
The last information day held in Kwazulu-Natal had a particularly strong turn-out, with 88 farmers and industry representatives attending the lecture at the Cedara College of Agriculture.
In many areas, brassica production by commercial or smallholder farmers occurs throughout the year, but depending on the local climate and irrigation conditions, some areas practice only summer or winter cropping. BSD has been reported to affect cabbage production in all seasons, depending on the area of production. In Brits (North West), where the disease was first noted, it is particularly a problem during winter, and crops planted after February are affected every year.
Monitoring in that area over the past five years has recorded disease pressures ranging from 50% to 95% in successive years. There has been no formal BSD monitoring in other areas in SA, but according to farmers attending these four events, BSD only really started to become problematic in their areas after 2014, with some areas becoming aware of the disease only in the past three years.
Most of the growers also reported much lower and inconsistent disease pressures (compared with the trend in Brits) for 2018 and 2019, i.e. 10%-80% in KwaZulu-Natal, 10%-50% in Limpopo, 3%-10% in Mpumalanga and 10%-40% in the Free State. Very worryingly, many farmers who have experienced successive seasons with 70%-90% disease incidence have said they are pulling out of the cabbage market due to the high crop and financial losses. From net house experiments in Brits between 2014 and 2016, it was clear that the BSD is transmitted by a flying insect vector.
In 2015, an insect transmission experiment was carried out to identify this vector. Collection of the predominant insect species occurring in BSD affected cabbage fields in Brits, and caging of these insects (i.e. aphids, plant and leaf hoppers and whiteflies) on cabbage seedlings resulted in the identification of aphids as the BSD insect vector. Only cabbage seedling cages with the aphid species Myzus persicae, developed typical BSD symptoms after 45 days. Consequently, an aphid monitoring study was done in Brits between 2016 and 2018, which identified a total of 36 different aphid species feeding on brassica crops.
The four predominant species were M. persicae, various Aphis spp., Rhopalosiphum maidis (corn aphid) and R. padi (Bird cherry-oat aphid). At the moment all of these species are considered potential BSD vectors. Aphids are small sap-sucking insects. There are about 4,700 species that vary greatly in their morphology (appearance) and life cycle characteristics. Around 400 species are considered agricultural pests of food and fibre crops, and about 250 species have been reported in South Africa. They are considered destructive pests because they weaken plants by sap depletion and most importantly, both nymphs and adults act as vectors for more than 100 plant viruses.
Aphids have a fairly complex life cycle, including both a summer (herbaceous) and winter (woody) host, a sexual and asexual reproduction phase, as well as the production of both a wingless and winged adult form at certain times of the year (fig 2 and fig 3). The wingless form is known to colonise summer crops and is responsible for secondary spreading of plant viruses within a field, whereas the winged form does not colonise plants, but is passively carried by wind over long distances and is responsible for primary infection in field crops. It is, in particular, the presence of the two aphid types, i.e. winged vs wingless, that is an important factor influencing the success of control strategies targeting aphid-transmitted diseases, like BSD.

Figure 2. Three morphological developmental stages of M. persicae, (A) nymphs and the wingless apterae and (B) the winged alate adult. (https://cabiplantwise.files.
wordpress.com/2017/02/gerenpeach- aphid.jpg)
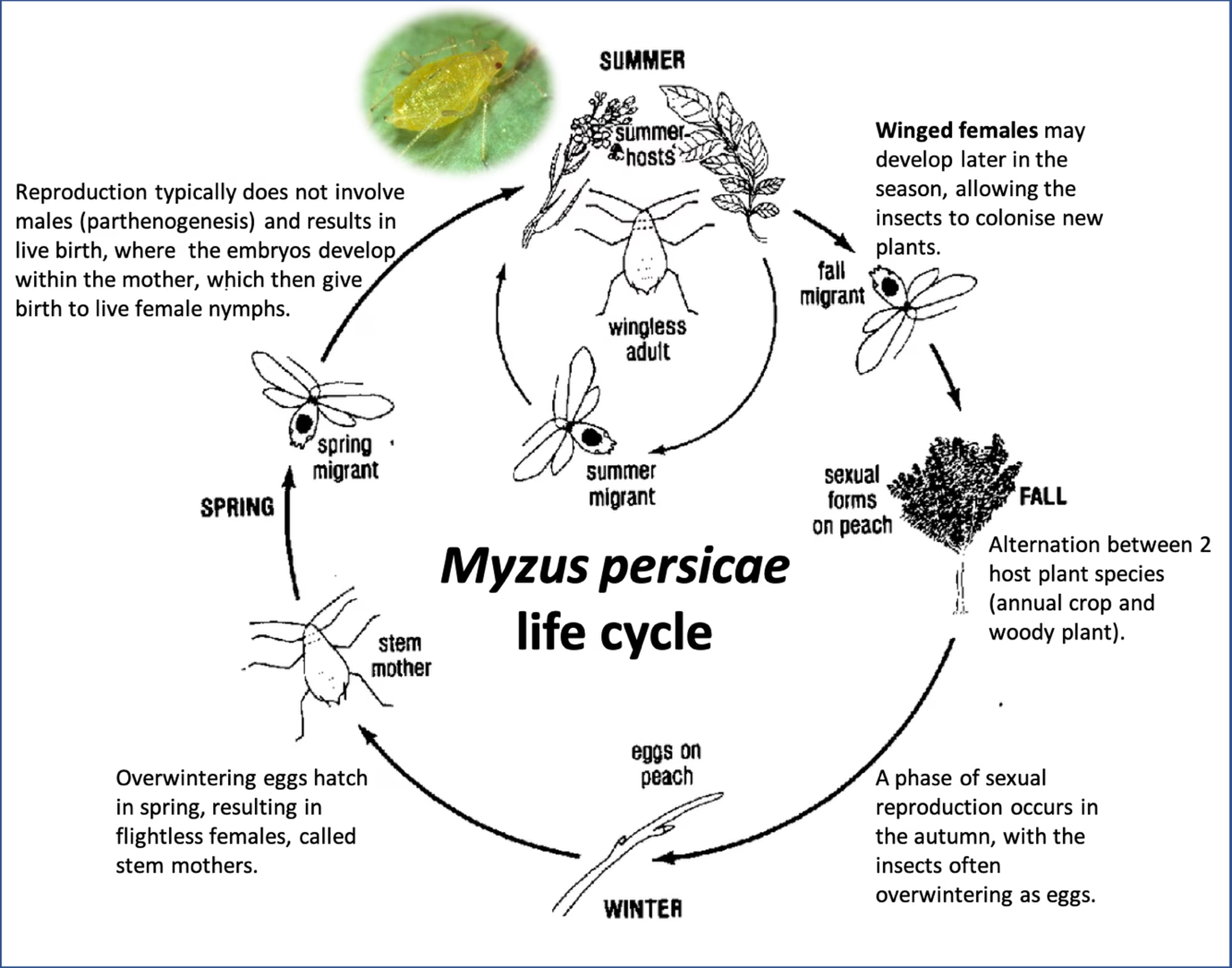
Figure 3. A representation of the life cycle of Myzus persicae, the green-peach aphid. The life cycle includes both a summer (herbaceous) host that includes plants from more than 40 families (i.e. vegetable crops in the families Solanaceae, Chenopodiaceae, Compositae, Brassicaceae, and Cucurbitaceae) and a winter (woody) host, particularly Prunus spp., especially peach, apricot and plum trees. Reproduction occurs both by a sexual and asexual reproduction phase in these two seasons, and the production of both a wingless and winged adult form occur at certain times of the year.
(Figure adapted from Radcliff, Ragsdale and Flanders, Management of aphids and leafhoppers, St Pileups Press, 1993)
Probably the most important and eagerly awaited information shared with the growers at the BSD information days was the research results regarding chemical control of the BSD. The effectiveness of several commercially available systemic insecticides to reduce aphid infestation and BSD infection was investigated during 2017 and 2018.
To reduce the cost impact on crop production, the evaluated insecticides were used in the form of two seed treatments (Sanokote (Dummypil), (thiamethoxam); Cruiser (thiamethoxam); and three seedling drenches (Actara (thiamethoxam); Kohinor and Confidor (imidacloprid). For the field trial, a susceptible cabbage cultivar was used and after treatment, it was transplanted into the open field in Brits, along with untreated plants as control. Aphid activity/infestation within the field was monitored weekly, by trapping winged aphids in yellow bucket traps and direct counting of winged and wingless aphids on the treated and untreated cabbage plants.
The results for the two years are summarised in fig 4. In 2017, symptoms of BSD appeared early in the season and were quite severe (fig 4A). The number of winged aphids counted in the traps and on the plants was relatively low throughout the season (5%), but the colonising, wingless aphids (75%) appeared soon after transplant and increased steadily in numbers over the season (8018 total aphid count) (fig 4E, F). Under these conditions, the use of systemic insecticides was highly effective in reducing aphid colonisation and BSD in the treated cabbage plants, with Actara (8%) and Kohinor (9.2%) significantly reducing BSD incidence in comparison with the untreated control (54%) (fig 4G).
In 2018, the number of winged aphids (62%) was much higher throughout the season (fig 4E, F), but there were fewer colonising wingless aphids (37%) (2445 total aphid count). In this year, the symptoms of BSD appeared much later in the season (fig 4B) and disease severity was much lower, probably due to the later occurrence of infection in 2018.
As a result, the systemic insecticides were less effective in controlling both the number of migrating wingless aphids counted on the crop plants and the BSD incidences (53%-69%) (fig 4G). At first, the presentation of such contrasting results regarding chemical control of BSD was highly disappointing to both the growers and industry representatives.
However, the results are a reflection of the complex nature of the BSD pathosystem. BSD is transmitted by aphids as its insect vector; consequently any fluctuation in the aphid populations (i.e. numbers, species or morphological types) will result in varied disease pressure. Indeed, disease monitoring in Brits over the past five years has revealed a bi-annual pattern in BSD incidence, with disease incidences of 90% and 50% every second year (Table 1).
This fluctuation in BSD incidence seemed to correlate with the fluctuation in total aphid numbers, as monitored between 2016 and 2018, i.e. higher aphid numbers result in higher disease pressure. The results have also suggested that the interaction between the insect vector and BSD incidence is more complex, because it is not only dependant on the aphid numbers in a season, but also the aphid types (winged vs wingless) and the timing of their appearance that play a role in disease incidence.
First, late season flights of winged aphids into the field will render insecticide application at the seed or seedling stage ineffective. Second, in seasons with high numbers of winged aphids throughout, additional follow-up treatments in the field will be required to extend the period of protection. Limiting the spread of BSD between fields (the migration of winged aphids from one field to nearby fields to inoculate a new field), is far more difficult, probably due to the inability of insecticides to instantly kill disease-carrying aphids and thereby prevent infection.
Research on Potato leaf roll virus (PLRV) transmission by M. persicae in potatoes has shown that even when the insecticide residues are still present within plants, virus-carrying winged aphids are not killed quickly enough to prevent virus transmission (by the time the aphid ingests the toxic active substance of the insecticide from the phloem sap, it has already transmitted the virus and only dies after transmission). Hence, special precautions must be taken against winged aphids when attempting to control BSD.
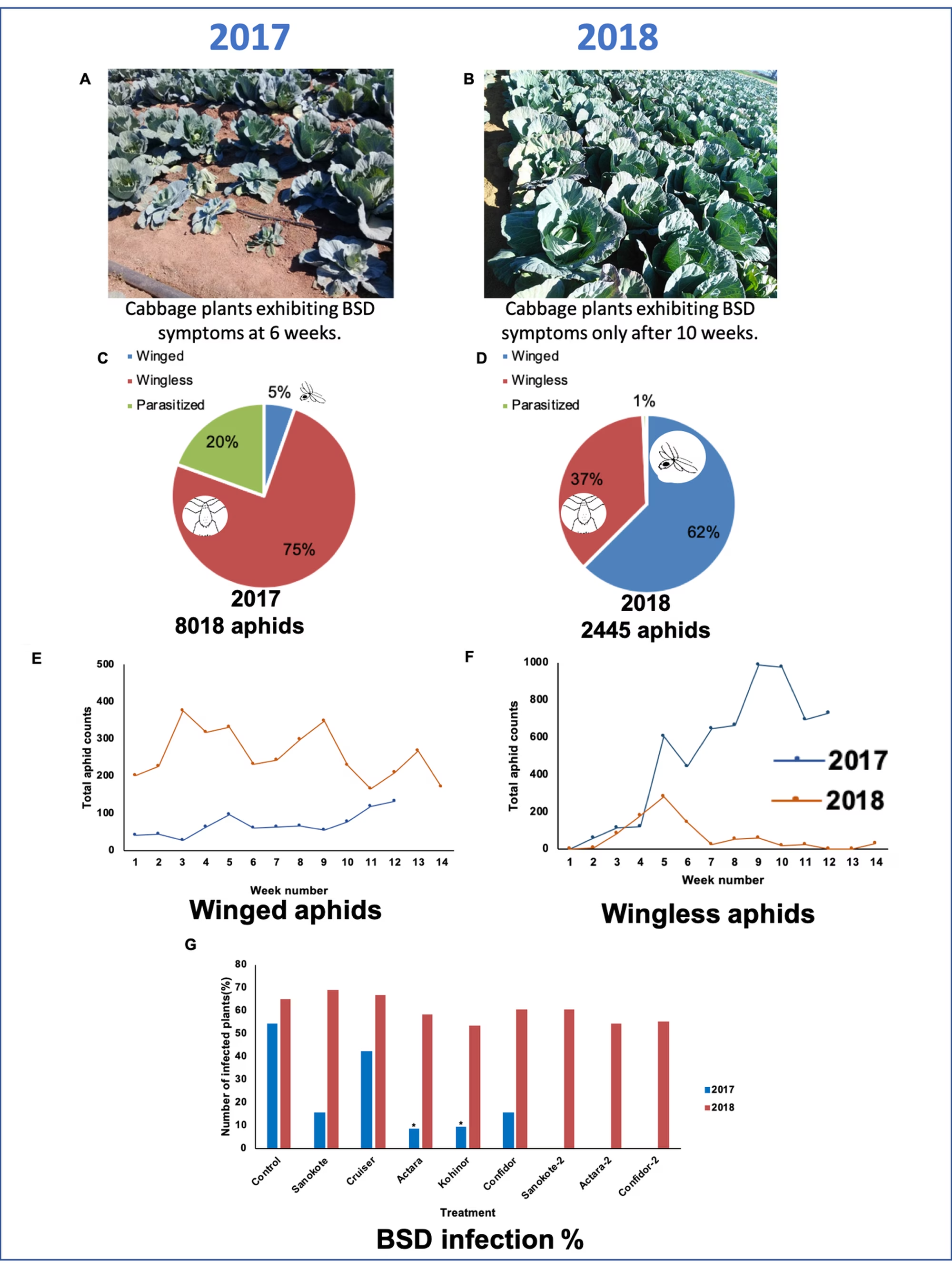
Figure 4. (A) In 2017, BSD associated symptoms such as stunting, yellowing and flattening of the leaves were observed as early as week six after transplanting. Most of the infected plants displayed a patch effect, i.e. adjacent plants within the same block exhibiting symptoms. (B) In comparison, at week 10 after transplant in 2018, the cabbage plants still appeared healthy, and the patch effect was not observed. (C) Total number of winged, wingless, and parasitised aphids counted directly on the cabbage plants. In 2017, 8018 aphids were counted and in 2018 (D), a total of 2445 aphids was counted. The total (E) winged aphid and (F) wingless aphid counts for 2017 (12-weeks) and 2018 (14-weeks) counted on a weekly basis, are indicated. (G) The BSD incidence (%) in treated and untreated cabbage plants in 2017 and 2018 is compared.
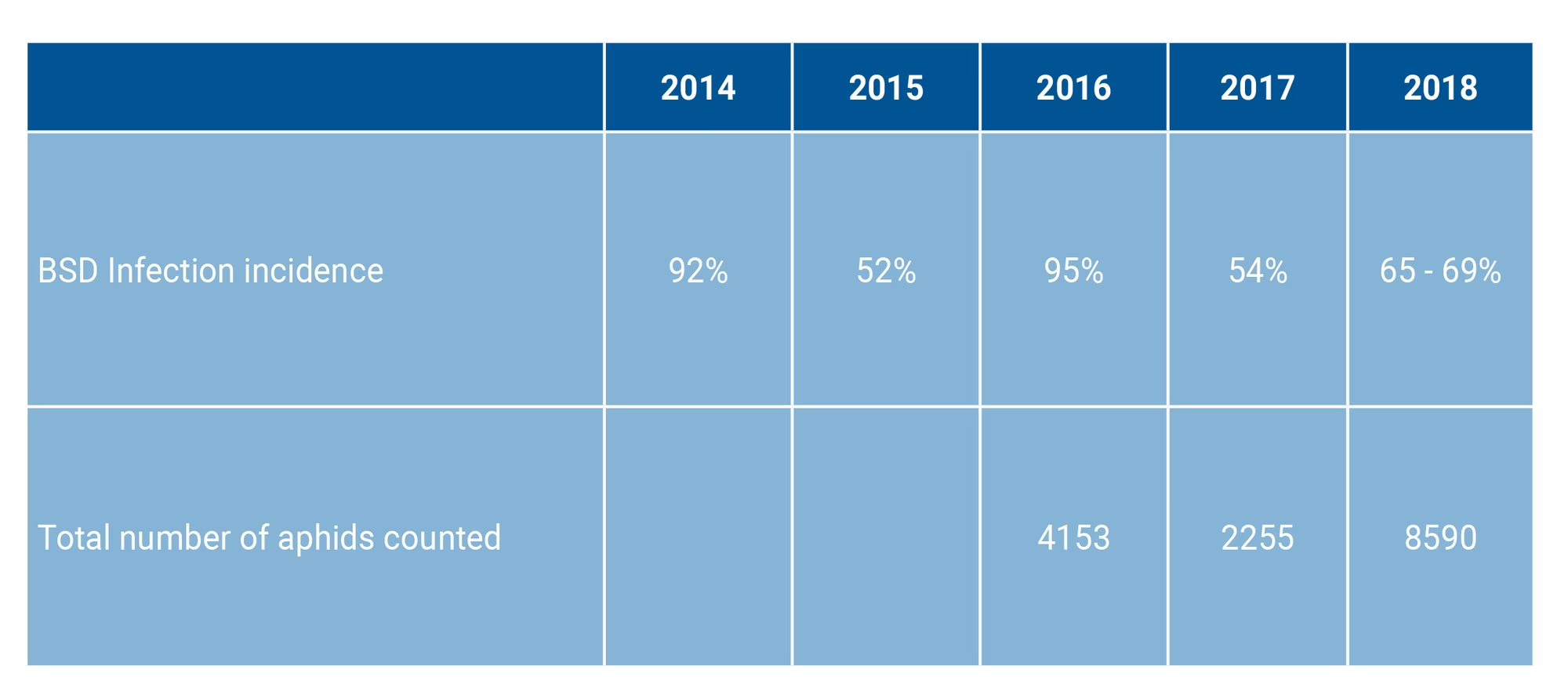
Table 1. A summary of BSD incidences and aphid activity monitored in Brits over five years.
In light of the information available on the BSD, the following advice is given to brassica growers attempting to control BSD infection. At a minimum, a seed or seedling treatment should be included in the nursery to prevent infection within the first six weeks. Our research has shown the disease is not seed transmissible and that infection occurs shortly after transplant into the field, as aphids start to feed and colonise the crop within the first day.
It is also very important that the seedling treatment is administered at least one day before transplant into the field (not as a seedling drench in trays adjacent to the field, on the day of transplant), to give the plant time to absorb and transport the systemic insecticide throughout the plant, before aphid exposure occurs in the field. Next, to extend the period of protection within the field, growers should speak to their pest control representative about an insecticide programme to control aphids throughout the season, particularly in seasons with high aphid numbers or high numbers of winged aphids. In seasons with high aphid numbers, particularly winged aphids, growers should attempt to reduce the numbers within the wider farming community (group effort), as aphids are transported over large distances by wind, making chemical control of BSD highly problematic.
Farmers are advised to go through their lands regularly and remove all plants showing BSD symptoms to reduce the amount of infected material. It might also be a good idea to remove or apply herbicide to any weedy areas around the land. It is likely wild brassica species growing as weeds around the crop are a symptomless reservoir for the BSD.
Research results have also highlighted the importance of aphid monitoring during the growth season, especially aphid flight activities. The use of aphid monitoring data, in the form of yellow bucket traps by growers themselves, or suction trap data to forecast aphid activity and flight patterns (recorded by the South African aphid monitoring system and currently used predominantly by potato and wheat growers), should be implemented to provide an early warning system about the risk for BSD infection in a specific area or season.